- Review
- Open access
- Published:
A review on hepatitis C virus: role of viral and host-cellular factors in replication and existing therapeutic strategies
Egyptian Liver Journal volume 12, Article number: 71 (2022)
Abstract
Background
Hepatitis C virus, a member of Flaviviridae is a single-stranded positive-sense RNA virus infecting 62–79 million people around the globe. This blood-borne virus is one of the leading causes of liver diseases worldwide. This review aims to identify novel potential genes linked to cellular host factors, as well as revise the roles of each gene in hepatitis C Virus infection. This review also aims to provide a comprehensive insight into therapeutic advancements against HCV.
Methods
For this review article, 190 articles were searched via PubMed Central, Bio-One, National Academy of Science, Google Scholar, and Worldwide Science. 0ut of these 190 studies, 55 articles were selected for this review. The inclusion of articles was done on the criteria of high citation and Q1 ranking.
Results
The information gathered from previously published articles highlighted a critical link between host-cellular factors that are important for HCV infection.
Conclusion
Although many advancements in HCV treatment have been made like DAAs and HTAs, the development of a completely effective HCV therapy is still a challenge. Further research on combinations of DAAs and HTAs can help in developing a better therapeutic alternative. Keywords: Hepatitis C virus, Replication cycle, Non-structural proteins, Host-cellular factors, Treatment strategies
Background
Hepatitis C virus (HCV) is a single-stranded blood-borne RNA virus infecting 62–79 million people around the globe. It is one of the leading causes of liver diseases worldwide. Almost 3% of the world population has the chronic infection of HCV that leads to fibrosis, cirrhosis, and eventually the carcinoma of hepatic cells in most cases [1]. In Pakistan, nearly 1 in every 20 individuals is a victim of this viral infection [2, 3]. To date, six different genotypes of HCV are reported. Due to the high mutation rate, all six genotypes further possess many different subtypes. Of these, four subtypes (1a, 1b, 2a, and 3a) are reported repeatedly across the globe, but the epidemics of others are still bound to specific geographic distributions [4] Genotype 4 is reported as the highly prevalent genotype in Middle East countries including Saudi Arabia and Central Africa but very little is known about its genotypic pandemic background at the subtype level in the region [5]. However, a recent virologic report on HCV has predicted the molecular phylogeny of 4a genotype with Egyptian prototype strain, while 1a isolates were closely related phylogenetically to North American and European countries [6]. A recent study in Iran has shown a remarkable growth of genotype 3a as a dominant infectious agent of HCV followed by 1a [7]. This high rate of mutations in the HCV virus has been a major hindrance in the development of an effective vaccine against its infection. The 9.6 kb genome of this enveloped virus consists of a single large open reading frame (ORF) and encodes 3 structural and 7 non-structural proteins. Structural proteins include core protein, envelope protein 1, and envelope protein 2. Non-structural proteins (NS) include p7, NS2, NS3 and NS4A, NS4B, NS5A, and NS5B (RNA-dependent RNA-polymerase [3].
Our genome is transcribed into two categories of transcriptional products, i.e., coding RNAs and non-coding RNAs. Coding RNAs are translated into proteins that play crucial biological functions in our body. Many of these protein-coding genes have been reported to be potential biomarkers for HCV infection. In this review, we have not only described the role of the coding region of the genome in HCV prognosis and diagnosis but have also highlighted the importance of the non-coding region. Non-coding RNAs once referred as “transcriptional noise”, are now being reported not only as good therapeutic targets but also as potential biomarkers. The non-coding RNAs play a significant regulatory role in the expression of gene networks [8]. Circular RNAs (circRNA) are a subset of specialized non-coding RNA. Interestingly, circRNAs have been reported to hinder the progress of tumor formation in HCV-related carcinoma by playing a regulatory role in cell division, growth, migration, and apoptosis. Thus, circular RNAs are not only potential therapeutic targets but can also serve as biomarkers for the diagnosis and prognosis of HCV infection and HCV-related hepatocarcinoma [9,10,11]. Moreover, MicroRNAs (miRNA) is another host factor reported to be associated with the progression of HCV infection [8, 12].
Methodology
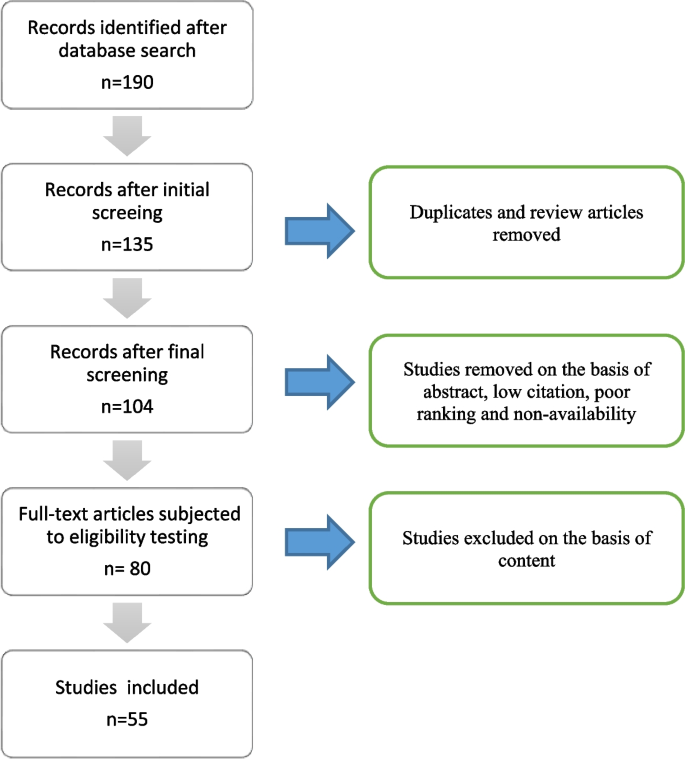
This article was prepared with the aim to review the role of viral and cellular host factors in infection of hepatitis C Virus. For this review article, 190 literature papers were searched via PubMed Central, Bio-One, National Academy of Science, Google Scholar, and Worldwide Science using the following keywords: ‘HCV’, ‘Life cycle of HCV’, ‘Translation of HCV genome’, ‘Host-cellular factors crucial for HCV infection’, ‘HCV receptors’, and ‘Immunity against HCV’. These 190 studies were then subjected to initial screening in which duplicated, and review articles were removed. Following an initial screening, the final screening was performed independently by two authors (Fatima Butt and Muhammad Shahid) on 135 studies. During the final screening, studies were subjected to our inclusion/exclusion criteria. Consequently, 104 articles were selected considering the following factors: (1) high citation and (2) Q1 ranking. Out of these 104 studies, 80 full-length articles were selected for eligibility testing. Subsequently, 55 articles were selected to be included in this review (Fig. 1).
Role of different viral and cellular factors in the life cycle of HCV
Life cycle
Attachment and entry
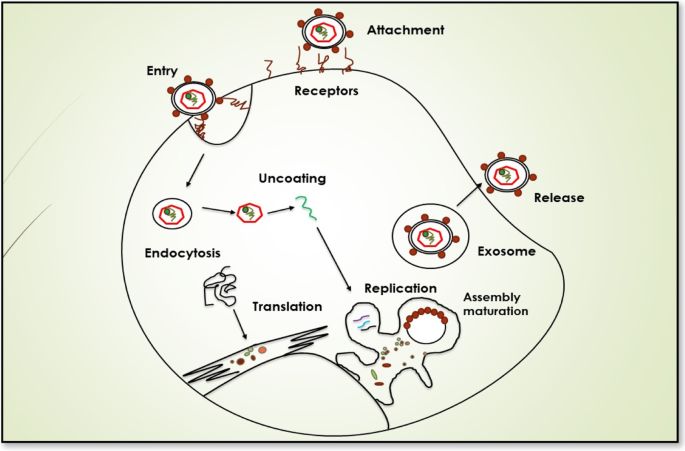
The presence of certain specific receptors and additional host entry factors at the hepatocytes make them susceptible and permissive cells for HCV infection as shown in Fig. 2. These blood-borne viral particles reach the basolateral surface of liver cells via blood flow after passing through sinusoids. The two transmembranes envelop proteins, E1 and E2, facilitating the binding of HCV to the host cell receptors. The outer surface of hepatocytes carries four receptors of HCV, i.e., the scavenger receptor class B type I (SCARB1), occluding (OCLN), cluster of differentiation 81(CD81), and claudin 1 (CLDN1) are present [13]. Along with these receptors, other additional factors like attachment factors glycosaminoglycans, low-density lipoproteins LDL-R receptors, Niemann-Pick C1-Like 1 (NPC1L1), and transferrin receptor 1 are also believed to contribute to the susceptibility of hepatocytes for HCV infection [1]. The association of HCV with lipoproteins and apolipoproteins makes its unique structure in the Flaviviridae family. This association helps in camouflaging HCV particles and prevents them from eliciting an immune response. However, the viral-lipoprotein association is also believed to facilitate the entry of HCV into host cells via LDL receptors only to onset a pathway leading to the degradation of these foreign viral particles by the host cell.
The first interaction for attachment between viral particles and hepatocytes is usually via SCARB1 which is an integral membrane protein that acts as one of the receptors for HCV particles. To date, several hypotheses regarding the HCV-SCARB1 association have been suggested. The earliest studies reported that viral glycoproteins are responsible for the initial interaction between HCV and host liver cells. The binding between the HVR1 region of E2 glycoproteins and the SCARB1 receptor of hepatocytes can be the first attachment signal stimulating the entry of the virus in host cells [14]. However, some modern studies also propose the involvement of associated apoprotein E in initial HCV-SCARB1 binding. Likewise, some other studies suggest that interaction between HRV1 and SCARB1 can also assist the binding of E2 with the CD81 receptor of host cells. CD81, a transmembrane protein, containing an extracellular domain for interaction with E2 of HCV particles is also considered one of the receptors for HCV particles [15, 16]. The HCV interaction with CD81 is considered to be a significant event in facilitating the viral entry into host cells [17]. CD81 is also believed to interact with another receptor of HCV known as CLDN1 [18]. CLDN1 is a tight junction protein that is expressed in many cells and tissues including liver cells. In hepatocytes, they play their role by separating bile from blood. The interaction between CD81 and CLDN1 is seemed to be assisted by EGFR (epidermal growth factor receptor). EGFR stimulates HRAS activation which results in CD81 diffusion, thus helping in the formation of the CD81-CLDN1 complex that enters the hepatocytes along with HCV particles [19]. Similarly, another tight junction protein, OCLN is one of the key receptors for HCV particles and plays a significant part in determining tissue tropism of HCV. However, the mechanism of OCLN action in the viral entry is still not known [14].
In addition to these receptors, some other host factors like factors glycosaminoglycans, Transferrin receptor 1, and NPC1L1 have also been shown to contribute to viral attachment and entry, but the exact mechanism of their role is still unknown. After attachment, the next step in the viral life cycle is the entry of these HCV particles into hepatocytes. These HCV particles enter hepatocytes via clathrin-dependent endocytosis. Following the entry into the cells in form of endosomes, pH-dependent fusion takes place between the endosomal membrane and viral envelope [20].
Translation and replication
The positive-strand RNA of HCV is released into the cytosol and its replication starts directly at ER via ribosomes of host hepatocytes. Essentially, only one large polyprotein is expressed from the single ORF of the viral genome which is then cleaved into 10 different proteins exploiting both host and viral proteases. The ORF carries the flanking regions 5′ and 3′ UTRs (Un-translated regions) that are essential for the translation and replication of viral RNA. 5′ UTR region includes an internal ribosome binding site (IRES) that instigates the translation of viral RNA into one large polyprotein. Proteolytic cleavage of this polyprotein results in the formation of ten different proteins. Among these 10 proteins, core protein, envelope protein 1, and envelope protein 2 are structural proteins while non-structural proteins include p7, NS2, NS3 and NS4A, NS4B, NS5A, and NS5B. Enzymes (non-structural protein) encoded by its genome viz. cysteine protease (NS2), serine protease (NS3-4A), helicase (NS3), and RNA-dependent RNA-polymerase (NS5B) are recruited during the replication process [21].
Signal peptide region E1 transports polyprotein to the ER membrane. Once the polyprotein reaches the membrane of ER, core protein is cleaved via subsequent host cell signal peptidase (SP) SP and signal peptide peptidase (SPP) cleavage. SP cleavage is important for the release of infectious viral particles from cells while SPP cleavage helps in viral displacement to lipid droplets. Owing to its cysteine proteolytic activity, NS2 is responsible for the cleavage of NS 2-3 junction. Likewise, NS3-assisted cleavage separates NS4A from NS3 and NS4B that ensues the NS3-4A association which can, in turn, modulate the cleavage of NS4B-5A and NS5B-5A junctions. After post-translational cleavages and polypeptide processing, non-structural HCV proteins are recruited for replication of viral RNA. A key event in this process is the formation of a negative-strand RNA intermediate by NS5B. After replication, the newly formed RNA molecules again go through the mechanisms of translation and replication and are ultimately packaged into capsids to form infectious viral particles. A specialized compartment called “membranous web” mainly composed of double-membrane vesicles (DMVs) is created in the cytosol for the replication of the HCV genome. The formation of this web is induced by viral proteins; however, the exact mechanism of induction is still not completely understood [21].
Several host-cellular factors are shown to be crucial for the replication of the HCV genome including micro-RNA 122, phosphatidyl-inositol-4-kinase-III (PI4KIII), ADP ribosylation factor GTPase activating protein 1 (ARFGAP1), and cyclophilin A (CypA) [22]. MicroRNA 122 is a liver enzyme that prevents the enzymatic degradation of viral RNA by attaching the Argonaute 2 enzyme at the 5′ UTR site thus stabilizing the viral genome. The interaction between PI4KIII and NS5A has also been shown to assist the maintenance of membranous web structure by mediating the accumulation of phosphatidylinositol-4-phosphate (PI4P) in this cytosolic web [23]. Furthermore, NS5A has also been reported to interact with CypA assisting in viral protein configuration and folding. Interestingly, inhibition of Cyp A has been shown to prevent the formation of double-membrane vesicles of the membranous web. Moreover, NS5A also interacts with ARFGAP1 for maintaining a high concentration of P1P4P at the membranous web [24]. Additionally, some other cellular molecules like proteins of intracellular transport, lipids including cholesterol, and vesicle-associated membrane proteins (VAP-A and VAP-B) have also been reported to favor the replication of HCV inside host cells.
Another key player in favoring HCV replication and translation is lipid droplets (LDs). LD-associated proteins, TIP47, and Rab18 stimulate LD interaction with NS5A and thus modulate viral replication [25]. Moreover, according to an ex vivo study, the presence of double-stranded RNA molecules of HCV adjacent to LD-rich sites illustrates the significance of LDs for the replication of the virus [26].
Assembly and release
Following genome translation and replication, assembly and release of infectious virions take place. The viral core proteins after undergoing SPP cleavage, are transported to LDs at which they get attached via their C terminal domain. The exact mechanism of HCV packaging and release is unclear. Consequently, different models have been proposed regarding its assembly and release. According to the most widely accepted model, the packaging of the viral genome into the capsids takes place in surrounding sites of LDs. Next, these nucleocapsids are trafficked to the ER from where they acquire their glycoprotein-containing envelope through budding. Another model proposes that E1 and E2 glycoproteins are dislocated to LD surrounding sites where viral particles get assembled. This dislocation is shown to be modulated by the interplay between NS2 and other viral proteins namely E1, E2, and p7 [27, 28]. Following assembly, HCV particles either undergo maturation or degradation depending upon their quality. Viral protein NS5A is crucial for virion assembly since phosphorylation of its C-terminal domain modulates viral packaging [29]. This C-terminal domain also interacts p7-NS2 complex [30]. Likewise, NS3-4 A complex also has been reported to be crucial for packaging HCV particles [31]. Moreover, mutations in p7, NS2, and NS5B proteins have been shown to cause improper packaging of viral particles elucidating the significance of these proteins in HCV morphogenesis [32].
Mature HCV particles exit the cells via very low density lipoprotein (VLDL) secretory pathway and circulate in the blood in close association with host lipoproteins. Thus, a host protein required for VLDL secretory pathway called MTP (microsomal triglyceride transfer protein) is also vital for the synthesis of infectious HCV particles. Similarly, another host protein Y-box-binding protein 1 (YB-1) interacts with the NS3-4A complex and has proved to be crucial for HCV particle synthesis. Additionally, two host enzymes namely DGAT1 (diacylglycerol acyltransferase 1) and PLA2GA4 (cytosolic phospholipase A2) are shown to play a key role in the assembly and release of HCV [33]. DGAT1 is essential for the interaction between viral core and lipid droplets while PLA2GA4 makes favorable changes in fluidity and curvature of membrane for proper assembly of HCV particles [34]. Moreover, glucosidase enzymes present in ER of the host modulate the proper folding of viral E1 and E2 proteins. Likewise, apoproteins (majorly apo E) have been reported to be crucial for the synthesis of infectious HCV particles [35]. Surprisingly, in vitro studies have also reported direct transfer of HCV infection from one cell to another known as cell-to-cell contact-mediated (CCCM) transfer for which all HCV receptors along with actin cytoskeleton are needed. However, this process remains unclear in vivo [36].
Therapeutic strategies
On account of the high mutation rate of this virus, no preventative vaccine is available for the hepatitis C virus. However, extensive research on therapeutic measures is being done. Currently, the most commonly used antiviral agents against HCV are direct antiviral drugs (DAAs) which target viral proteins. The most widely targeted proteins include NS3-4 complex owing to its proteolytic activity and NS5B protein due to its RNA-dependent RNA polymerase activity. The first DAA that was licensed against HCV used NS3-4 complex as the target. A study in 2019 has shown that DAAs have improved survival rates in patients having HCV-related cirrhosis. Chronic infections by all 6 major genotypes of HCV have been reported to be treated safely and efficiently using a combination of two DAAs targeting NS3-4 complex (Glecaprevir) and NS5A (pibrentasvir) [36].
Sofosbuvir (SOF) is a very efficient and effective pan-genotypic drug, especially for 1-4 HCV genotypes. It has great potential to act as an antiviral agent and has suitable pharmacokinetic properties [37]. This drug is safe for consumption and there is minimal risk of drug resistance. It is a NS5B nucleotide inhibitor that prevents the formation of HCV nucleotide (RNA) synthesis by the termination of RNA chains [38].
Likewise, another pan-genotypic drug, MAVYRET (combination of Glecaprevir and Pibrentasvir) has been approved by FDA in 2017 for treating chronic hepatitis C infections. EPCLUSA (combination of sofosbuvir and velpatasvir) is another pan-genotypic drug that has been approved for treating adults infected with chronic HCV infection. VOSEVI, a remarkable drug which is the combination of drugs from three separate antiviral groups, have been approved in 2017 for HCV genotypes 1–6 [37].
However, genetic diversity among variants due to the high mutation rate of HCV is a major challenge to the broad-term efficacy of DAAs. Moreover, serious side-effects and the development of resistance in patients are also some of the major shortcomings of DAAs. One of the possible reasons for this resistance could be the cell-to-cell-mediated transferability of HCV [39].
Viral genotype assessment is required in nations without pan-genotypic programs to customize medication and provide affordable therapies [40]. The drug named HARVONI which is a combination of ledipasvir and sofosbuvir was approved in 2014 for treatment of HCV genotype 1 in patients having liver cirrhosis. It is more effective drug as compared to traditional Ribavirin or peg-interferone therapy. It is a non-invasive treatment method having less side effects, high SVR rates, and the brief length of therapy [40]. Zepatier is an excellent drug for treatment of HCV genotypes 1 and 4 infection. It is a combination of grazoprevir and elbasvir. It yields high SVR rates and is favorable in terms of safety and efficacy. Patients with liver cirrhosis can also be treated using Zepatier [41]. Daclatasvir is an efficient antiviral agent used for the treatment of HCV genotypes 2 and 3. It is an inhibitor of NS5A protein. Daclatasvir is used in combination with sofosbuvir for treating HCV infections with SVR rates of 90% in patients having cirrhosis and > 90% for patients without cirrhosis. This drug has high safety and tolerability properties and can effectively treat HCV infections [42].
A combination of sofosbuvir and velpatasvir is approved for the treatment of patients infected with genotypes 5 and 6 for 4 months. High SVR rates are achieved in case of patients receiving treatment and for those who have not been treated before. Patients having cirrhosis are also effectively treated using this drug [43].
Now with the advancements in tissue culture models for HCV, host factors have also been shown to be potential targets against HCV infection that resulted in the development of host targeting agents (HTAs). An effective way to control HCV infection is to inhibit the entry of HCV in hepatocytes. This can be achieved by targeting host receptors and additional entry factors. One of the potential targets is the SCARB1 receptor against which ITX 5061 is available as HTA. However, a mutation in E2 glycoprotein has been reported as a result of long-term use of ITX 5061, indicating the possibility of HTA resistance in patients. Remarkably, neutralizing antibodies have also been proved to be effective as in HCV clearance. In addition to entry factors, other factors of host required at later steps of HCV cycle can also be used as targets for HTAs like CypA, apo E, miR-122, MTTP, PI4KIIIα, and DGAT1. For instance, Avasimibe, an inhibitor of Apo B and Apo E secretion, is a clinically approved HTA and can be used against all 6 major genotypes of HCV [39]. Likewise, ASP5286, a potential inhibitor of HCV has been discovered that targets cyclophilin [44].
The non-coding RNAs (ncRNAs) is a novel class of potential genes that can serve as potential targets for drug development or discovery. These genes not only regulate the expression and functioning of normal genes but also aid in disease progression. Understanding the mechanism of action of these genes and their classification can aid in designing customized therapies for the treatment of HCV and other infections [8].
Circular RNAs (circRNA) are a subtype of non-coding RNAs that despite their low abundance, are very stable molecules and are found to be associated with the progression of several diseases. In the case of HCV infection, some circRNAs are found to be upregulated while others are reported to be downregulated. One example is of circPSD3 whose deregulation was found to be associated with the pathogenesis of HCV [9]
Moreover, downregulation of circ-ZEB1.33 is found to be associated with HCC progression. On contrary, some circRNAs hinder the progression of HCV-related carcinoma, e.g., circFBLIMI and circSETD3. Thus, the potential of circRNAs as biomarkers can be evaluated by analyzing their expression upon HCV infection [10].
Interestingly a study has reported upregulation of circCMTM3 in HCC cells. This circ RNA has been shown to regulate the expression of EZH2 and thus can serve as a therapeutic target in HCV infection [11]
MicroRNA ‘miR-122’ is abundantly present and highly expressed in liver cells. It facilitates the synthesis of HCV RNA by efficiently binding with 5′-UTR of HCV RNA. Targeting this microRNA, the replication of HCV RNA can be inhibited. Miravirsen, an anti-miR-122 is developed to prevent miRNA-122 role in promoting HCV infection [8]. Miravirsen injections have been given to hepatitis patients and a substantial amount of decreased miRNA levels have been observed in the plasma of these patients [12].
MicroRNA (MiR-155) is responsible for cancer formation due to its oncogenic potential. It causes inflammation leading to the formation of tumors at sites. The levels of MiR-155 are upregulated in the case of hepatocellular carcinoma. MiR-155 promotes cell migration, cell invasion and cell proliferation in hepatic cells (hepatocytes) [8].
Some other novel genes include TAF1 and HNF4A can serve as potential biomarkers for the assessment of liver fibrosis. These can also be targeted to design therapy for liver treatment. The CALM2 gene levels are found to be inversely proportional to the degree of liver fibrosis. Upregulation of the CALM2 genes can be helpful for designing liver cancer treatment [45].
A potential vaccine candidate against HCV could be a disulfide motif of broadly neutralizing antibodies that interacts with E2 protein [46]. Similarly, neutralizing antibodies against the AR3 epitope of HCV have been reported to promote viral clearance [47]. Interestingly, it has been proposed that combinations of different neutralizing antibodies can be an effective and broad-term therapy against the hepatitis C virus. Moreover, a peptide vaccine containing 6 different epitopes has been shown to induce a very strong immune response in mice models. Likewise, antibodies against host factor OCLN has been reported to completely inhibit HCV infection in a mouse [48]. A study has reported that recombinant E1E2 glycoproteins of the HCV envelope can induce an insufficient anti-body response in humans and non-human primates [49]. In addition to entry factors, other factors of host required at later steps of the HCV cycle can also be used as targets for HTAs like CypA, apo E, miR-122, MTTP, PI4KIIIα, and DGAT1 [49].
Conclusion
During the last two decades, extensive research on the hepatitis C virus has provided significant information regarding its structure and life cycle that not only revealed its molecular nature but also gave insight into better therapeutic strategies. Although many advancements in HCV treatment have been made like DAAs and HTA s, the development of a completely effective HCV therapy is still a challenge. Further research on combinations of DAAs and HTAs can help in developing a better therapeutic alternative. Furthermore, many viral and host-cellular factors involved in different stages of the HCV life cycle have been discovered; however, the complete mechanism is still unknown. Extensive studies in this area would not only contribute to the knowledge concerning HCV infection but would also help in the development of novel and modern treatment strategies against this blood-borne virus.
Availability of data and materials
N/A
References
Stanaway JD et al (2016) The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 388(10049):1081–1088
Al Kanaani Z et al (2018) The epidemiology of hepatitis C virus in Pakistan: systematic review and meta-analyses. R Soc Open Sci 5(4):180257
Morozov VA, Lagaye S (2018) Hepatitis C virus: Morphogenesis, infection and therapy. World J Hepatol 10(2):186
Mostafa A et al (2016) Excess mortality rate associated with hepatitis C virus infection: a community-based cohort study in rural Egypt. J Hepatol 64(6):1240–1246
Khan A et al (2017) Tracing the epidemic history of hepatitis C virus genotypes in Saudi Arabia. Infect Genet Evol 52:82–88
AlMalki WH et al (2021) Virological surveillance, molecular phylogeny, and evolutionary dynamics of hepatitis C virus subtypes 1a and 4a isolates in patients from Saudi Arabia. Saudi J Biol Sci 28(3):1664–1677
Rezaee N, Babaeekhou L, Ghane M (2020) Hepatitis C virus in Iran; transmission routes, growth in 3a genotype distribution, and lack of liver marker relation with genotypes. Int J Res Med Sci 25
Wang X et al (2021) MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 70(4):784–795
Chen T-C et al (2020) Host-derived circular RNAs display proviral activities in Hepatitis C virus-infected cells. PLoS Pathog 16(8):e1008346
Song C et al (2019) The competing endogenous circular RNA ADAMTS14 suppressed hepatocellular carcinoma progression through regulating microRNA-572/regulator of calcineurin 1. J Cell Physiol 234(3):2460–2470
Lin T et al (2019) Silencing Of hsa_circ_0008450 represses hepatocellular carcinoma progression through regulation of microRNA-214-3p/EZH2 Axis. Cancer Manag Res 11:9133–9143
Qadir MI et al (2020) RNA therapeutics: identification of novel targets leading to drug discovery. J Cell Biochem 121(2):898–929
Pileri P et al (1998) Binding of hepatitis C virus to CD81. Science 282(5390):938–941
Ploss A et al (2009) Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457(7231):882–886
Bankwitz D et al (2010) Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J Virol 84(11):5751–5763
Scarselli E et al (2002) The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J 21(19):5017–5025
Fénéant L, Levy S, Cocquerel L (2014) CD81 and hepatitis C virus (HCV) infection. Viruses 6(2):535–572
Harris HJ et al (2010) Claudin association with CD81 defines hepatitis C virus entry. J Biol Chem 285(27):21092–21102
Iwamoto M et al (2020) The machinery for endocytosis of epidermal growth factor receptor coordinates the transport of incoming hepatitis B virus to the endosomal network. J Biol Chem 295(3):800–807
Blanchard E et al (2006) Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol 80(14):6964–6972
Moradpour D, Penin F (2013) Hepatitis C virus proteins: from structure to function. Hepatitis C virus: from molecular virology to antiviral therapy, pp 113–142
Germain M-A et al (2014) Elucidating novel hepatitis C virus–host interactions using combined mass spectrometry and functional genomics approaches. Mol Cell Proteomics 13(1):184–203
Reiss S et al (2011) Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe 9(1):32–45
Li H et al (2014) Hepatitis C virus NS5A hijacks ARFGAP1 to maintain a phosphatidylinositol 4-phosphate-enriched microenvironment. J Virol 88(11):5956–5966
Salloum S et al (2013) Rab18 binds to hepatitis C virus NS5A and promotes interaction between sites of viral replication and lipid droplets. PLoS Pathog 9(8):e1003513
Targett-Adams P, Boulant S, McLauchlan J (2008) Visualization of double-stranded RNA in cells supporting hepatitis C virus RNA replication. J Virol 82(5):2182–2195
Jirasko V et al (2010) Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog 6(12):e1001233
Ma Y et al (2011) Hepatitis C virus NS2 protein serves as a scaffold for virus assembly by interacting with both structural and nonstructural proteins. J Virol 85(1):86–97
Tellinghuisen TL, Foss KL, Treadaway J (2008) Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog 4(3):e1000032
Scheel TK et al (2012) Analysis of functional differences between hepatitis C virus NS5A of genotypes 1–7 in infectious cell culture systems. PLoS Pathog 8(5):e1002696
Phan T et al (2011) The acidic domain of hepatitis C virus NS4A contributes to RNA replication and virus particle assembly. J Virol 85(3):1193–1204
Gouklani H et al (2012) Hepatitis C virus nonstructural protein 5B is involved in virus morphogenesis. J Virol 86(9):5080–5088
Herker E et al (2010) Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat Med 16(11):1295–1298
Menzel N et al (2012) MAP-kinase regulated cytosolic phospholipase A2 activity is essential for production of infectious hepatitis C virus particles. PLoS Pathog 8(7):e1002829
Weller R et al (2017) Hepatitis C virus strain-dependent usage of apolipoprotein E modulates assembly efficiency and specific infectivity of secreted virions. J Virol 91(18):e00422–e00417
Liu Z, He JJ (2013) Cell-cell contact-mediated hepatitis C virus (HCV) transfer, productive infection, and replication and their requirement for HCV receptors. J Virol 87(15):8545–8558
Desnoyer A et al (2016) Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol 65(1):40–47
Ahmed H et al (2018) Safety and efficacy of sofosbuvir plus velpatasvir with or without ribavirin for chronic hepatitis C virus infection: a systematic review and meta-analysis. J Infect Public Health 11(2):156–164
Xiao F et al (2014) Hepatitis C virus cell-cell transmission and resistance to direct-acting antiviral agents. PLoS Pathog 10(5):e1004128
Aboras SI et al (2021) A review on analytical strategies for the assessment of recently approved direct acting antiviral drugs. Crit Rev Anal Chem:1–23
Papudesu C, Kottilil S, Bagchi S (2017) Elbasvir/grazoprevir for treatment of chronic hepatitis C virus infection. Hepatol Int 11:152–160. https://doi.org/10.1007/s12072-016-9761-2
Manolakopoulos S et al (2016) Safety and efficacy of daclatasvir in the management of patients with chronic hepatitis C. Ann Gastroenterol 29(3):282
Feld JJ et al (2015) Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 373(27):2599–2607
Makino T et al (2020) Discovery of ASP5286: A novel non-immunosuppressive cyclophilin inhibitor for the treatment of HCV. Bioorg Med Chem Lett 30(16):127308
Ji D et al (2019) Identification of TAF1, HNF4A, and CALM2 as potential therapeutic target genes for liver fibrosis. J Cell Physiol 234(6):9045–9051
Flyak AI et al (2020) An ultralong CDRH2 in HCV neutralizing antibody demonstrates structural plasticity of antibodies against E2 glycoprotein. Elife 9
Merat SJ et al (2019) Cross-genotype AR3-specific neutralizing antibodies confer long-term protection in injecting drug users after HCV clearance. J Hepatol 71(1):14–24
Shimizu Y et al (2018) Monoclonal antibodies against occludin completely prevented hepatitis C virus infection in a mouse model. J Virol 92(8):e02258–e02217
Wakita T et al (2005) Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11(7):791–796
Acknowledgements
All authors contributed to the main writing and editing of the manuscript. All authors have read and approved the final manuscript.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
FB, MH, and MS analyzed and interpreted patient data and drafted the manuscript. FT, IA, SA RB, RN, and MI participated, coordinated, and analyzed the clinical data. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A
Consent for publication
All authors contributed to the main writing and editing of the manuscript. All authors have read and approved the final manuscript.
Competing interests
All the authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Butt, F., Shahid, M., Hassan, M. et al. A review on hepatitis C virus: role of viral and host-cellular factors in replication and existing therapeutic strategies. Egypt Liver Journal 12, 71 (2022). https://doi.org/10.1186/s43066-022-00232-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-022-00232-w